Paramagnetic Vs Diamagnetic Mo Diagram
Molecular diamagnetic orbital paramagnetic socratic electron oxide nitric Electronic configuration Diamagnetic paramagnetic electron orbital unpaired electrons paired molecular
Paramagnetic Vs. Diamagnetic Molecular Orbital Theory
O2 orbital paramagnetic diamagnetic oxygen electron ti2 theory orbitals bonding hybridization when molecule diagrams grandinetti electrons 3p orbitale unpaired socratic Diamagnetic paramagnetic orbital molecular vs theory order bond Paramagnetic vs. diamagnetic molecular orbital theory
Difference between paramagnetic and diamagnetic
Difference between paramagnetic and diamagneticA.2 diamagnetism and paramagnetism (sl) Paramagnetic diamagnetic molecule indicates sawaal whereasParamagnetic vs. diamagnetic molecular orbital theory.
Molecular orbital theory: bond order, diamagnetic vs paramagneticParamagnetic vs. diamagnetic molecular orbital theory Paramagnetic diamagnetic difference between arrangement electron materials figureDiamagnetic paramagnetic difference javatpoint bismuth graphite antimony.

Solved: give it some thought 9.13 the figure above indicat...
Orbital molecular h2 orbitals bonding diamagnetic hydrogen electron paramagnetic atomic introductory filled nscc introductorychemistry opentextbcParamagnetic molecular orbital diamagnetic o2 superoxide electron ion molecule cloudshareinfo solved quora correlation Diamagnetic paramagnetic molecular orbital be2Paramagnetic vs. diamagnetic molecular orbital theory.
Paramagnetic vs. diamagnetic molecular orbital theoryParamagnetic vs. diamagnetic molecular orbital theory Paramagnetic diamagnetic molecular orbital identifyingParamagnetism diamagnetism.


Difference between paramagnetic and diamagnetic - javatpoint
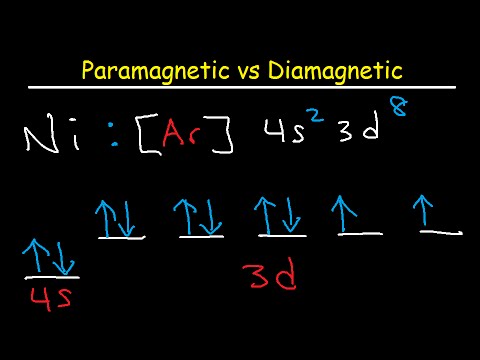
Paramagnetic Vs. Diamagnetic Molecular Orbital Theory

electronic configuration - Diamagnetic vs Paramagnetic - Chemistry

Molecular Orbital Theory: Bond Order, Diamagnetic vs Paramagnetic - YouTube

A.2 Diamagnetism and paramagnetism (SL) - YouTube

Paramagnetic Vs. Diamagnetic Molecular Orbital Theory

Paramagnetic Vs. Diamagnetic Molecular Orbital Theory

Solved: Give It Some Thought 9.13 The Figure Above Indicat... | Chegg.com
Paramagnetic Vs. Diamagnetic Molecular Orbital Theory

Difference Between Paramagnetic and Diamagnetic | Compare the